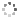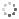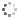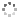issue contents
August 2018 issue
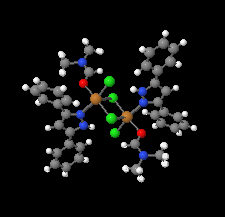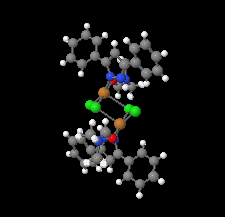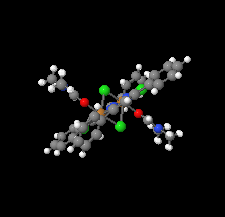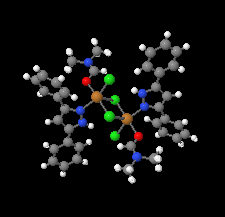
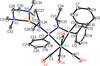
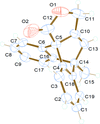
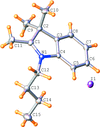
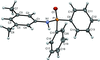
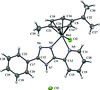
Cover illustration: Pyrazole-based ligands are ubiquitous in the literature for their ability to build mono-metal complexes and molecules containing multiple metal centers. In particular, 3,5-diphenylpyrazole and its derivatives have been used to form numerous copper complexes with the metal in either the 1+ or 2+ oxidation states. The compound di-![[mu]](/logos/entities/mu_rmgif.gif) -chlorido-bis[chlorido(dimethylformamide-
-chlorido-bis[chlorido(dimethylformamide-![[kappa]](/logos/entities/kappa_rmgif.gif) N)(3,5-diphenyl-1H-pyrazole-
N)(3,5-diphenyl-1H-pyrazole-![[kappa]](/logos/entities/kappa_rmgif.gif) N2)copper(II)], Cu2(
N2)copper(II)], Cu2(![[mu]](/logos/entities/mu_rmgif.gif) -Cl)2Cl2(3,5-diphenyl-1H-pyrazole)2(DMF)2, where DMF is N,N-dimethylformamide, consists of two five-coordinate CuII ions with a distorted square-pyramidal geometry connected via two
-Cl)2Cl2(3,5-diphenyl-1H-pyrazole)2(DMF)2, where DMF is N,N-dimethylformamide, consists of two five-coordinate CuII ions with a distorted square-pyramidal geometry connected via two ![[mu]](/logos/entities/mu_rmgif.gif) -Cl anions. The coordination environment of each CuII ion is completed by a terminal chloride anion, a nitrogen-coordinated 3,5-diphenyl-1H-pyrazole molecule, and a DMF molecule. Intermolecular hydrogen bonding between the molecules generates a two-dimensional sheet. See: Naugle, Keller, Zeller & Zaleski [IUCrData (2017). 3, x181186].
-Cl anions. The coordination environment of each CuII ion is completed by a terminal chloride anion, a nitrogen-coordinated 3,5-diphenyl-1H-pyrazole molecule, and a DMF molecule. Intermolecular hydrogen bonding between the molecules generates a two-dimensional sheet. See: Naugle, Keller, Zeller & Zaleski [IUCrData (2017). 3, x181186].
inorganic compounds


 access
accessmetal-organic compounds


 access
access

 access
access

 access
access

 access
access

 access
access

 access
accessorganic compounds


 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
accessaddenda and errata
 access
access

 journal menu
journal menu










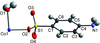
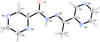
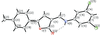
![[publCIF]](/logos/authorchecklist11.gif)