issue contents
November 2023 issue
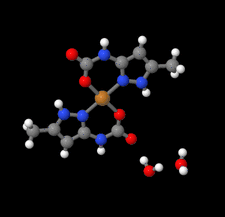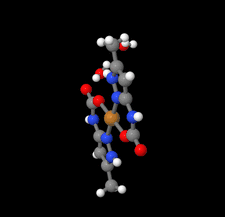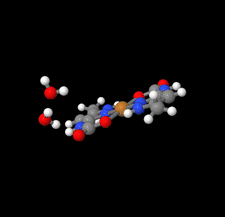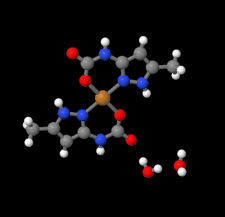
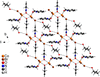
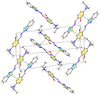
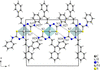
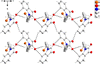
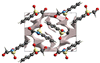
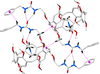
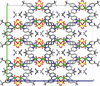
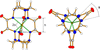
Cover illustration: High levels of greenhouse gas emissions, in particular carbon dioxide, are considered the primary source of the global warming that our planet is experiencing. Among several strategies being explored for the mitigation and control of these emissions are processes that separate, capture and store CO2 through the so-called carbon capture and sequestration materials (CCS). The stored gases can then be used in reactions leading to materials useful in other different processes such as the synthesis of catalysts or pharmaceutical compounds. This article presents the structure of a novel Cu-carbamato complex, bis[N-(5-methyl-1H-pyrazol-3-yl-κN2)carbamato-κO]copper(II) tetrahydrate, obtained in a reaction driven by absorption of CO2 present in the environment. See Sirenko, Kuzevanova, Vynohradov, Naumova & Shova [Acta Cryst. (2023). E78, 988–992].
research communications























































 journal menu
journal menu