issue contents
March 2022 issue
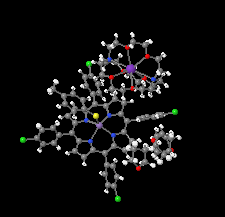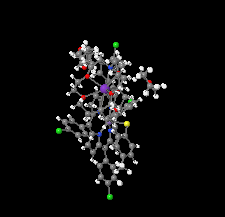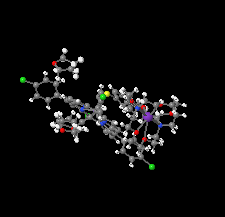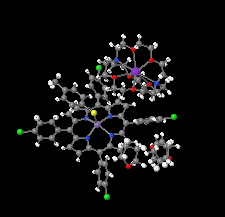
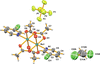
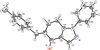
Cover illustration: In ([2.2.2]cryptand)potassium (4-methylbenzenethiolato)[5,10,15,20-tetrakis(4-chlorophenyl)porphyrinato]manganate(II) tetrahydrofuran disolvate, the MnII cation is coordinated by four pyrrole N atoms (Np) of the porphyrin ring and one S atom of the apical 4-methylbenzenethiolate ligand with the average Mn—Np and the apical Mn—S bond lengths being 2.160 (9) and 2.4642 (8) Å, respectively. Two tetrahydrofuran solvent molecules and a potassium cation chelated inside a [2.2.2]cryptand (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane) are also present. See: Yuan & Li [IUCrData (2022). 7, x220241].
metal-organic compounds


 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
accessorganic compounds


 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 journal menu
journal menu











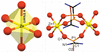
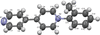
![[publCIF]](/logos/authorchecklist11.gif)