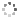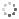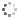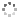issue contents
September 2020 issue
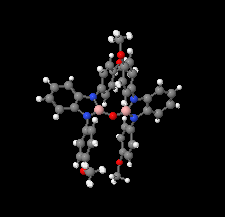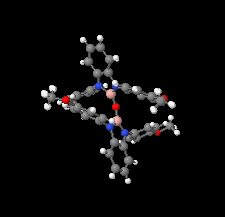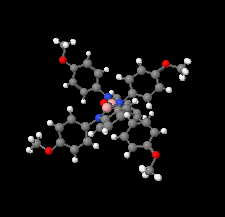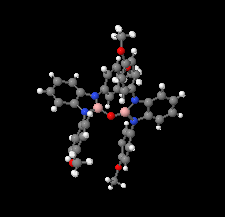
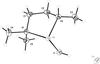
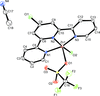
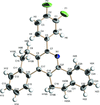
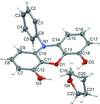
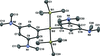
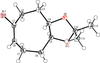
Cover illustration: The field of cooperative catalysis has given scientists the ability to access more complex molecular transformations using cheaper, readily available metals. The compound 2,2'-oxybis[1,3-bis(4-methoxyphenyl)-2,3-dihydro-1H-benzo[d][1,3,2]diazaborole] was synthesized using elements from the main group of the periodic table, which are cheaper and more accessible than the traditionally used transition metals. It has a pincer-like orientation formed by an oxygen single-atom bridge connected to two Lewis-acidic boron centers. The diamine moieties bound to the boron atoms provide redox-active sites, which give the structure the electron equivalents that boron lacks while also modulating the steric environment. The pincer shape might allow the compound to use the boron atoms and the redox-active ligands to create a binding pocket for coordination and bridging of a small molecule substrate. See: Mallard, Kennedy, Rudman, Greenwood, Nicoleau, Angle, Torquato, Gau, Carroll & Anstey [IUCrData (2020). 5, x201248].
inorganic compounds


 access
accessmetal-organic compounds


 access
access

 access
access

 access
access

 access
access

 access
access

 access
accessorganic compounds


 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 journal menu
journal menu











![[publCIF]](/logos/authorchecklist11.gif)