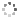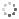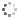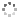issue contents
August 2020 issue
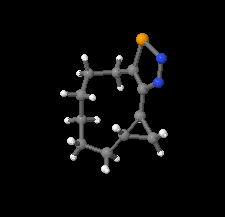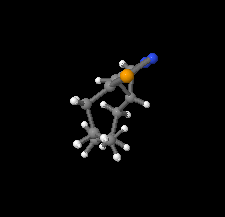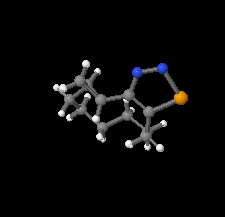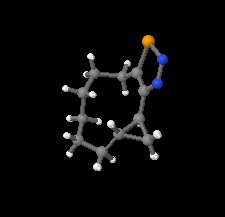
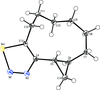
Cover illustration: 1,2,3-Selenadiazoles are synthesized from SeO2-oxidation of semicarbazones and are important intermediates for the synthesis of medium-sized, heterocyclic and strained cycloalkynes. The centrosymmetric crystal structure of rac-12-selena-13,14-diazatricyclo[9.3.0.02,4]tetradeca-11,13-diene is built up from alternating strands of (R,R)- and (S,S)-enantiomers. These strands, which propagate along the c-axis direction, are composed of homochiral molecules related to each other by twofold screw axes. The shape of the molecule is an almost planar unit around the selenadiazole ring with a hexamethylene chain as an arched handle.See: Detert & Schollmeyer [IUCrData (2020). 5, x201081].
metal-organic compounds


 access
access

 access
accessorganic compounds


 access
access

 access
access

 access
access

 access
access

 journal menu
journal menu










![[publCIF]](/logos/authorchecklist11.gif)