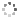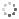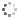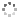issue contents
June 2020 issue
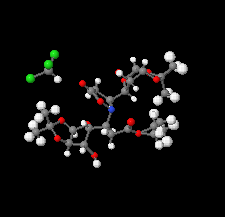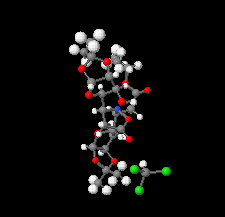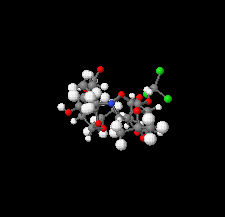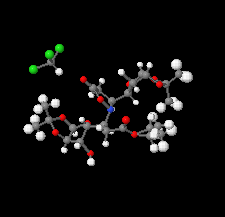
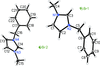
Cover illustration: Ethyl (3S)-3-[(3aR,5R,6S,6aR)-6-hydroxy-2,2-dimethyltetrahydrofuro[4,5-d][1,3]dioxol-5-yl]-3-{(3S)-3-[(3aR,5R,6S,6aR)-6-hydroxy-2,2-dimethyltetrahydrofuro[4,5-d][1,3]dioxol-5-yl]-5-oxoisoxazolidin-2-yl}propanoate chloroform monosolvate was obtained as a product of a double aza-Michael addition of hydroxylamine on a Chiron with a known absolute configuration. The enantiopure compound crystallized as a chloroform solvate, in space group P1, and diffraction data were collected at room temperature with Ag K![[alpha]](/logos/entities/alpha_rmgif.gif) radiation. The Flack parameter refined to x = -0.01 (16); however, the Flack and Watkin 2AD plot clearly shows that differences between Friedel opposites (the D component of the plot) do not carry any reliable information about resonant scattering of Cl atoms, and are rather dominated by random and systematic errors. This example illustrates that while using Ag K
radiation. The Flack parameter refined to x = -0.01 (16); however, the Flack and Watkin 2AD plot clearly shows that differences between Friedel opposites (the D component of the plot) do not carry any reliable information about resonant scattering of Cl atoms, and are rather dominated by random and systematic errors. This example illustrates that while using Ag K![[alpha]](/logos/entities/alpha_rmgif.gif) radiation (
radiation (![[lambda]](/logos/entities/lambda_rmgif.gif) = 0.56083 Å), scatterers heavier than Cl should be present in a chiral crystal in order to determine confidently the absolute structure of the crystal. See: Amaro Hernández, Rodríguez Tzompantzi, Dávila García, Meza-León & Bernès [IUCrData (2020). 5, x200788].
= 0.56083 Å), scatterers heavier than Cl should be present in a chiral crystal in order to determine confidently the absolute structure of the crystal. See: Amaro Hernández, Rodríguez Tzompantzi, Dávila García, Meza-León & Bernès [IUCrData (2020). 5, x200788].
metal-organic compounds


 access
access

 access
accessorganic compounds


 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 journal menu
journal menu










![[publCIF]](/logos/authorchecklist11.gif)