issue contents
August 2024 issue
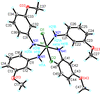
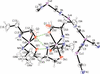
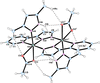
Cover illustration: Copper cyanide networks are of continuing interest because of the wide variety of different networks found and the interesting and potentially useful magnetic or photoluminescent properties shown by some of them. Poly[tris(2-aminobutan-1-ol)copper(II) [hexakis-μ2-cyanido-κ12C:N-tetracopper(I)] bis(2-aminobutan-1-olato)aquacopper(II) monohydrate] was obtained serendipitously during attempts to synthesize mixed-valence CuCN networks with the use of the base 2-amino-1-butanol. Instead of the expected structure type, with CN bridging CuI and CuII atoms, {[Cu(C4H11NO)3][Cu4(CN)6]·[Cu(C4H10NO)2(H2O)]·H2O}n, was obtained, which is made up of diperiodic anionic CuICN networks and two independent CuII complexes that are not covalently bonded to the networks. See: Corfield & Salvi [IUCrData (2024). 8, x240845].
metal-organic compounds


 access
access

 access
access

 access
access

 access
access

 access
access

 access
accessorganic compounds


 access
access

 access
access

 access
access

 access
access

 access
access

 access
access
 journal menu
journal menu










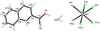


![[publCIF]](/logos/authorchecklist11.gif)