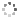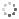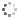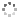issue contents
July 2024 issue
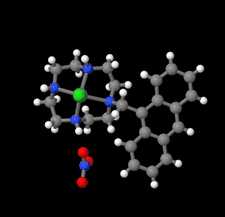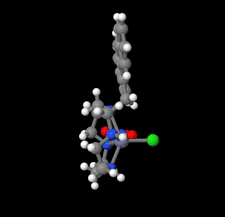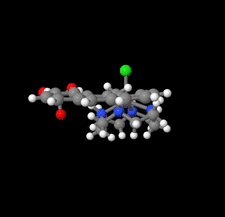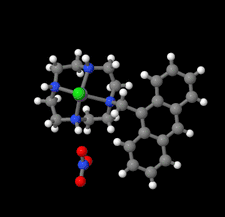
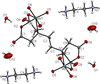
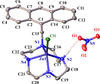
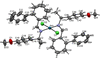
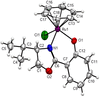
Cover illustration: Complexes of 1,4,7,10-tetraazacyclododecane (cyclen or [12]aneN4) derivatives with ZnII have been used as biological probes to elucidate the intrinsic roles of ZnII in enzyme models such as phosphatase, alcohol dehydrogenase, and β-lactamase. Cyclen conjugated with the anthracenyl methyl group has been developed as a fluorescent chemosensor for detecting pH and transition-metal cations in aqueous solution. In this context, the crystal structure of the salt, [1-(anthracen-9-ylmethyl)-1,4,7,10-tetraazacyclododecane]chloridozinc(II) nitrate is reported. The crystal structure comprises a [Zn(C23H30N4)Cl]+ complex cation and a nitrate anion. The coordination environment around the ZnII atom is slightly distorted square-pyramidal. The anthracene group attached to cyclen contributes to the crystal packing through intermolecular T-shaped π interactions. Additionally, the nitrate anion participates in intermolecular N—H⋯O hydrogen bonds with cyclen. See: Ichimaru, Sugiura, Kato, Kondo, Kurihara, Jin, Imai & Kurosaki [IUCrData (2024). 8, x240665].
metal-organic compounds


 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access
 journal menu
journal menu










![[publCIF]](/logos/authorchecklist11.gif)