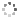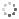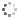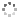issue contents
May 2024 issue
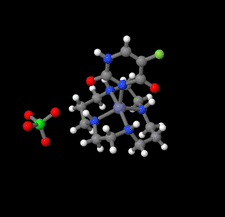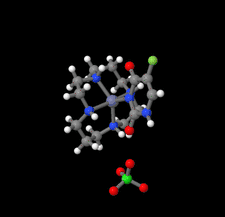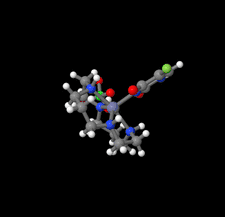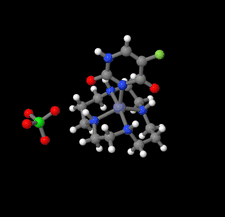
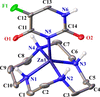
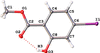
Cover illustration: In the structure of the complex (5-fluoro-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-1-ido-κN1)(1,4,8,11-tetraazacyclotetradecane-κ4N)zinc(II) perchlorate, the zinc(II) ion forms coordination bonds with the four nitrogen atoms of cyclam (1,4,8,11-tetraazacyclotetradecane or [14]aneN4) as well as with the nitrogen atom of a deprotonated 5-fluorouracil ion (FU−). Cyclam adopts a trans-I type conformation within this structure. The coordination structure of the zinc(II) ion is a square pyramid with a distorted base plane formed by the four nitrogen atoms of the cyclam. FU− engages in intermolecular hydrogen bonding with neighboring FU− molecules and with the cyclam molecule. See: Ichimaru, Kato, Jin, Kurihara & Kurosaki [IUCrData (2024). 8, x240431]
inorganic compounds
Na2(Fe2/3Te4/3)O6 was obtained under hydrothermal conditions and adopts the ilmenite (FeTiO3) structure type.
metal-organic compounds
The crystal structure of the title complex is stabilized by intermolecular O—H⋯Cl hydrogen bonds, forming
![[R_{2}^{2}]](/x/issues/2024/05/00/bx4028/teximages/bx4028fi1.svg)
(18) ring motifs.
In the structure of the title complex, the zinc(II) ion forms coordination bonds with the four nitrogen atoms of cyclam as well as with the nitrogen atom of a deprotonated 5-fluorouracil ion. Cyclam adopts a trans-I type conformation within this structure. The coordination structure of the zinc(II) ion is a square pyramid with a distorted base plane formed by the four nitrogen atoms of the cyclam.
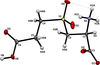
The crystal structure of the title complex features O—H⋯O hydrogen bonds, which form
![[R_{2}^{2}]](/x/issues/2024/05/00/wm4213/teximages/wm4213fi1.svg)
(8),
![[R_{4}^{4}]](/x/issues/2024/05/00/wm4213/teximages/wm4213fi2.svg)
(16),
![[R_{4}^{4}]](/x/issues/2024/05/00/wm4213/teximages/wm4213fi2.svg)
(20) and
![[R_{4}^{4}]](/x/issues/2024/05/00/wm4213/teximages/wm4213fi2.svg)
(22) ring motifs.
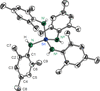
The molecular structure of Sn(NHMes)4 is defined by a central tin(IV) atom that is coordinated by four NHMes groups in a distorted tetrahedral arrangement.
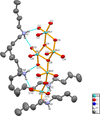
(C8H20N)8[Mo10O34] comprises a centrosymmetric decamolybdate polyanion linked through N—H⋯O hydrogen bonds to dibutylammonium counter-cations.
organic compounds
The title compound forms a sheet structure of dimers exhibiting intra- and intermolecular hydrogen bonds.
The molecule is a zwitterion, with a protonated α-amino group and a deprotonated α-carboxyl group. Within the crystal, the molecules are linked by a system of hydrogen bonds formed by both the protonated and deprotonated carboxylic groups, and the protonated ammonium group.
The N—H group of the title compound does not form a hydrogen bond due to steric hindrance.
In the crystal of the title compound, the molecules are connected through C—H⋯O hydrogen bonds, generating [100] chains, which are crosslinked by weak π–π stacking interactions.



 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access
 journal menu
journal menu










![[publCIF]](/logos/authorchecklist11.gif)