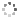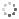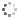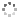issue contents
October 2020 issue
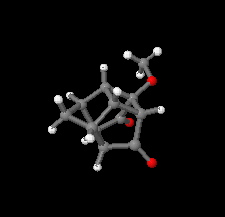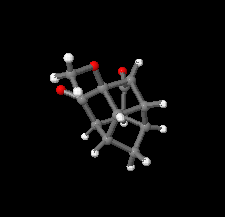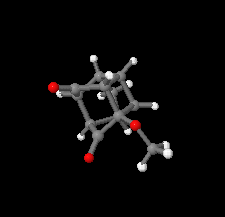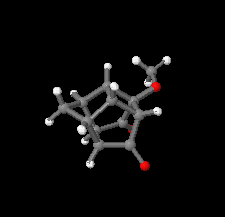
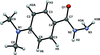
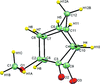
Cover illustration: Polycyclic cage hydrocarbons act as valuable synthons in pharmaceutical and medicinal chemistry and are also useful candidates in energetic materials. Most of the cage systems display functions in supramolecular chemistry and asymmetric catalysis. Oxygenated cage compounds show significant biological activity. The crystal structure of the methoxy-substituted Cookson's dione derivative, 7-methoxypentacyclo[5.4.0.02,6.03,10.05,9]undecane-8,11-dione, C12H12O3, at 150 K has monoclinic (P21/c) symmetry. The pentacycloundecane cage compound is composed of four five-membered rings, a planar four-membered ring and a six-membered ring in a boat conformation fused into a closed strained-cage framework. All of the five-membered rings adopt an envelope conformation. A slight distortion is observed after substitution with a methoxy group compared to Cookson's dione skeleton. See: Kotha, Ansari & Cheekatla [IUCrData (2020). 5, x201380].
inorganic compounds


 access
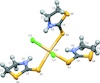
accessCrystal structure of Al2.95Cr0.59, a phase closely related to the η-phase in the binary Al–Cr system
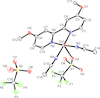
metal-organic compounds


 access
access

 access
access

 access
access

 access
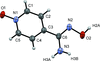
accessorganic compounds


 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 journal menu
journal menu











![[publCIF]](/logos/authorchecklist11.gif)