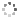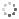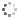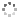issue contents
December 2020 issue
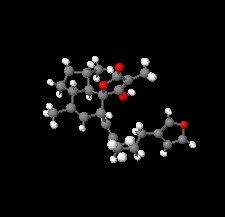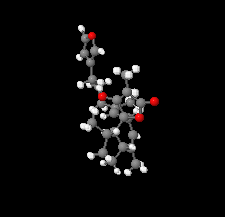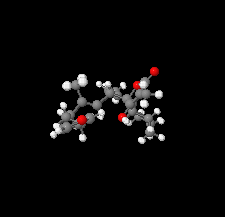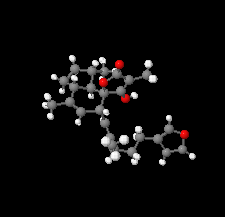
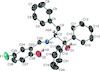
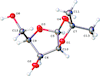
Cover illustration: The title compound ircinianin, systematic name (2S,3'S,3a'R,5'R,7a'R)-5'-[(E)-5-(furan-3-yl)-2-methylpent-1-en-1-yl]-3-hydroxy-3',4,7'-trimethyl-1',2',3',3a',5',7a'-hexahydro-5H-spiro[furan-2,4'-inden]-5-one, belongs to the sesterterpene tetronic acid compound family and was isolated from the marine sponge Ircinia wistarii. These chemical scaffolds are pharmacologically relevant, since they represent a new class of glycine receptor modulators. See: Majer, Schollmeyer, Koch & Gross [IUCrData (2020). 5, x201578].
metal-organic compounds
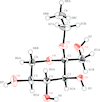
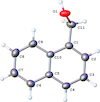
The manganese(III) atom of the title compound is coordinated by one bromide and two benzo[h]quinolin-10-olate ligands and exhibits a distorted square-pyramidal coordination environment.
organic compounds
The title compound was prepared and fully characterized. The salicylaldehyde alcohol is engaged in an intramolecular O—H⋯N hydrogen bond with the imine nitrogen.
In the title compound, the salicylaldehyde alcohol group is engaged in an intramolecular O—H⋯N hydrogen bond with the imine nitrogen atom, while the tertiary alcohol is engaged in a weak intermolecular O—H⋯F hydrogen bond with an adjacent molecule.
The title compound ircinianin belongs to the sesterterpene tetronic acid compound family and was isolated from the marine sponge Ircinia wistarii. These chemical scaffolds are pharmacologically relevant, since they represent a new class of glycine receptor modulators.
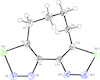
The title compound crystallizes in a non-symmetrical conformation with a dihedral angle between the heterocycles of 45.0 (3)° and a nearly strain-free tetramethylene tether.
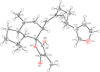
The title compound was synthesized by the dehydrative condensation of α-L-sorbose and ethanol.
In the title compound, the pentofuranose ring has a twisted conformation while the other five-membered ring has an envelope conformation; the two hydroxy groups C are involved in an infinite network of O—H⋯ O bonds, forming a layer parallel to the (001) plane.
The molecules of naphthalen-1-ylmethanol are linked by O—H⋯O hydrogen bonds, generating infinite chains propagating parallel to the [100] direction.
The title compound, C21H22O2, crystallizes in its keto-form. The molecules are connected via weak C—H⋯O interactions, forming infinite chains perpendicular to the [001] axis.



 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 journal menu
journal menu










![[publCIF]](/logos/authorchecklist11.gif)