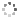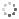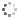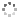issue contents
April 2021 issue
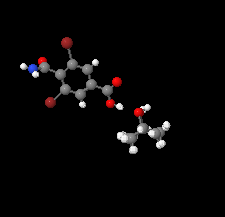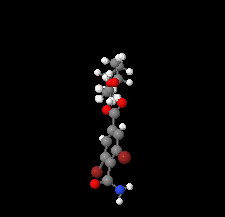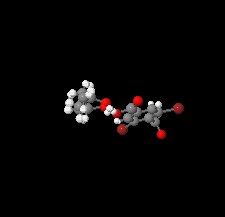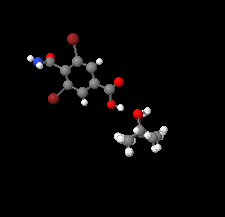
Cover illustration: In the crystal of 3,5-dibromo-4-carbamoylbenzoic acid 2-propanol monosolvate, the acid molecules form inversion dimers by pairwise N—H⋯O hydrogen bonds between carbamoyl groups and the carboxyl and carbamoyl groups link to form head-to-tail inversion dimers. The 2-propanol hydroxyl group interposes between adjacent head–tail pairs, resulting in C33(10) chains of hydrogen bonds propagating along [100]. The best-fit planes of the carbamoyl group and benzene ring are inclined by 88.26 (11)°. This is a greater inclination than was previously reported with CH3, Cl, F or H in place of the Br atoms, although those analogues did not have a para carboxyl group. See: Noland, Herzig, Fox & Tritch [IUCrData (2021). 6, x210391].
metal-organic compounds


 access
accessorganic compounds


 access
access5-[(1,3-Dimethyl-5-oxo-2-sulfanylideneimidazolidin-4-ylidene)amino]-2-methylisoindoline-1,3-dione


 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 journal menu
journal menu











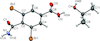
![[publCIF]](/logos/authorchecklist11.gif)