issue contents
November 2021 issue
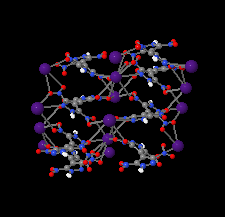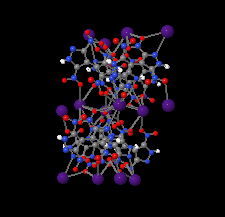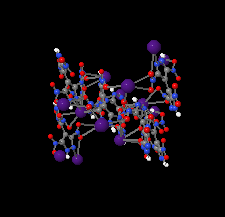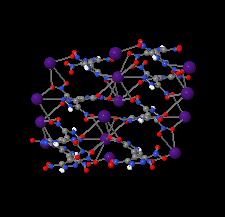
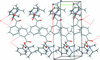
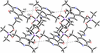
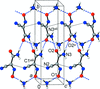
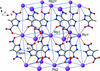
Cover illustration: Energetic materials attract a great deal of attention because of their potential applications. At the same time, green approaches to pyrotechnic formulations are gaining interest in the synthesis of new flame colourants. In this contribution, Domasevitch & Ponomarova present the structures of isostrutural Rb and Cs complexes containing a nitro-substituted pyrazol/pyrazolate that exhibit the CsCl topology when considering their ionic networks. Purple and orange coloured flames, respectively, are produced when these cations burn. The crystal engineering approach to the design of these materials will be useful in the synthesis of other polynitro-based energetic compounds. See: Domasevitch & Ponomarova [Acta Cryst. (2021). E77, 1109–1115].
Jerry P. Jasinski tribute


research communications


















































 journal menu
journal menu