issue contents
January 2021 issue
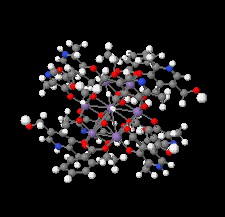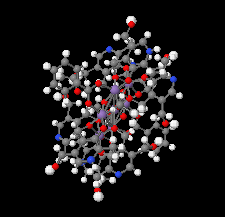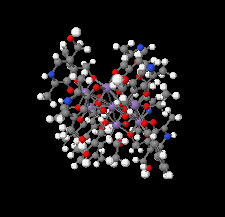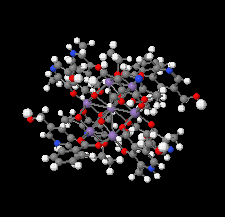
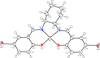
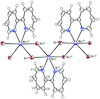
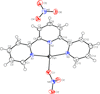
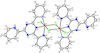
Cover illustration: Work in the area of synthesis of polynuclear manganese complexes and their magnetic properties led to the synthesis and crystallization of the compound di-μ-benzoato-di-μ-ethanolato-tetrakis[μ3-5-(hydroxymethyl)-2-methyl-4-(oxidomethyl)pyridin-1-ium-3-olato]tetrakis[μ3-5-(hydroxymethyl)-2-methyl-4-(oxidomethyl)pyridin-3-olato]di-μ3-oxido-heptamanganese(II,III) ethanol octasolvate, [Mn7(C8H9NO3)4(C8H10NO3)4(C2H5O)2(C7H5O2)2O2]·8C2H5OH. The compound was synthesized by the reaction of Mn(C6H5COO)2 with pyridoxine (PNH2, C8H11NO3) followed by the addition of tetramethylammonium hydroxide (TMAOH). The core of this centrosymmetric complex is a cage-like structure consisting of six MnIII ions and one MnII ion bound together through Mn—O bonds. The compound crystallizes in hydrogen-bonded layers formed by O—H⋯N hydrogen bonds involving the aromatic amine group of the ligand PN2− with the neighboring O atoms from the PNH− ligand. The crystal structure has large voids present in which highly disordered solvent molecules (ethanol) sit. See: Saha, Padgett, LeMagueres, Moncur & Onajobi [IUCrData (2020). 5, x201578].
metal-organic compounds


 access
access

 access
access

 access
access

 access
access

 access
access

 access
accessorganic compounds


 access
access

 access
access

 access
access

 access
accessaddenda and errata
 access
access

 journal menu
journal menu










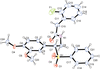
![[publCIF]](/logos/authorchecklist11.gif)