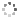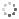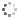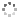issue contents
May 2017 issue
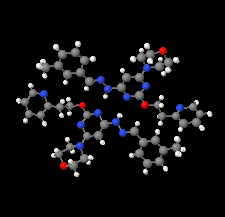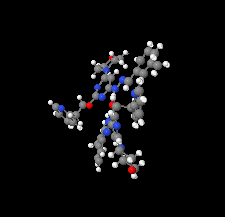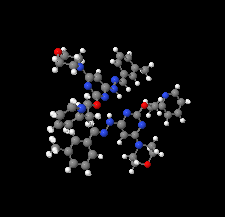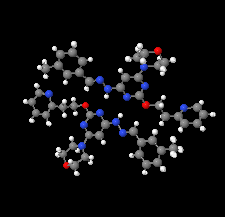
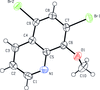
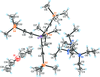
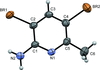
Cover illustration: Apilimod {systematic name: N-[(E)-(3-methylbenzylidene)amino]-6-(morpholin-4-yl)-2-[2-(pyridin-2-yl)ethoxy]pyrimidin-4-amine}, a molecule of interest for its antiviral properties, was acquired from several different commercial vendors. Analysis of several commercial batches had led to some ambiguity over the exact structure. In order to remove any ambiguity, the structure was confirmed by X-ray crystallography. In addition, the NMR spectra are provided as reference material for future investigations. See: Morris, Moore & Thomas [IUCrData (2017). 2, x170693].
metal-organic compounds


 access
accessorganic compounds


 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 access
access

 journal menu
journal menu













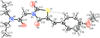
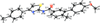



![[publCIF]](/logos/authorchecklist11.gif)